PRODUCT IDENTIFICATION
H.S. CODE
TOXICITY
CLASSIFICATION
GENERAL DESCRIPTION OF FATTY ACID
PHYSICAL AND CHEMICAL PROPERTIES
pale yellow liquid
AUTOIGNITION
NFPA RATINGS
REFRACTIVE INDEX
> 110 C
GENERAL DESCRIPTION & APPLICATIONS
pale yellow liquid
|
|
|
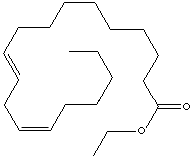
| LINOLEIC ACID ETHYL ESTER | ||
|
PRODUCT IDENTIFICATION |
||
| CAS NO. | 544-35-4 |
|
| EINECS NO. | 208-868-4 | |
| FORMULA | C17H31COOC2H5 | |
| MOL WT. | 308.50 | |
|
H.S. CODE |
3823.11 | |
| SMILES |
|
|
|
TOXICITY |
||
| SYNONYMS | Ethyl linolate; | |
| 9,12-linoleic acid, ethyl ester; (Z,Z)-9,12-Octadecadieneoic acid, ethyl ester; | ||
|
CLASSIFICATION |
||
|
GENERAL DESCRIPTION OF FATTY ACID |
||
| Fatty Acids are aliphatic carboxylic acid with varying hydrocarbon lengths at one end of the chain joined to terminal carboxyl (-COOH) group at the other end. The general formula is R-(CH2)n-COOH. Fatty acids are predominantly unbranched and those with even numbers of carbon atoms between 12 and 22 carbons long react with glycerol to form lipids (fat-soluble components of living cells) in plants, animals, and microorganisms. Fatty acids all have common names respectively lilk lauric (C12), MyrIstic (C14), palmitic (C16), stearic (C18), oleic (C18, unsaturated), and linoleic (C18, polyunsaturated) acids. The saturated fatty acids have no double bonds, while oleic acid is an unsaturated fatty acid has one double bond (also described as olefinic) and polyunsaturated fatty acids like linolenic acid contain two or more double bonds. Lauric acid (also called Dodecanoic acid) is the main acid in coconut oil (45 - 50 percent) and palm kernel oil (45 - 55 percent). Nutmeg butter is rich in myristic acid (also called Tetradecanoic acid ) which constitutes 60-75 percent of the fatty-acid content. Palmitic acid(also called Hexadecylic acid ) constitutes between 20 and 30 percent of most animal fats and is also an important constituent of most vegetable fats (35 - 45 percent of palm oil). Stearic acid ( also called Octadecanoic Acid) is nature's most common long-chain fatty acids, derived from animal and vegetable fats. It is widely used as a lubricant and as an additive in industrial preparations. It is used in the manufacture of metallic stearates, pharmaceuticals, soaps, cosmetics, and food packaging. It is also used as a softener, accelerator activator and dispersing agent in rubbers. Oleic acid (systematic chemical name is cis-octadec-9-enoic acid) is the most abundant of the unsaturated fatty acids in nature. | ||
|
PHYSICAL AND CHEMICAL PROPERTIES |
||
| PHYSICAL STATE |
pale yellow liquid | |
| MELTING POINT | ||
| BOILING POINT | ||
| SPECIFIC GRAVITY | 0.87 - 0.88 | |
| SOLUBILITY IN WATER | ||
| pH | ||
| VAPOR DENSITY |
| |
|
AUTOIGNITION |
| |
|
NFPA RATINGS |
Health: 1 Flammability: 1 Reactivity: 0 | |
|
REFRACTIVE INDEX |
| |
| FLASH POINT |
> 110 C | |
| STABILITY | Stable under ordinary conditions. Light sensitive | |
|
GENERAL DESCRIPTION & APPLICATIONS |
||
| Linoleic acid is a polyunsaturated fatty acid (18:2 omega-6) occurring as a glyceride in drying oils; boils at 229 C. It is a common fatty acid produced in plants. It is not synthesized in human. It is considered as an essential fatty acid in human nutrition. It is used in feeds, nutrient food additives and medicines. It is used as a drying agent for protective coatings. It is used in manufacturing emulsifying agents, soaps and paints. Ethyl linolate has imporved stability and absorption by skin. End application of Linoleic acid esters includes cosmetics and personal care products. | ||
| SALES SPECIFICATION | ||
| APPEARANCE |
pale yellow liquid |
|
| CONTENT | 98.0% min | |
| TRANSPORTATION | ||
| PACKING | | |
| HAZARD CLASS | Not regulated | |
| UN NO. | ||
| OTHER INFORMATION | ||
|
|
||
|
|
|